Semipermeable Membranes and Osmotic Flow
So what’s Osmosis Anyway?
Osmosis is the process in which a liquid passes through a membrane whose pores permit the passage of solvent molecules, but are too small for the larger solute molecules to pass through.
What, you don’t get it? Let’s break osmosis down to its parts to get a grasp on it.
First, we’ll make our solution. We start with a boring old cup of water. To spice things up, we’ll call water the “solvent” — which is convenient, because that’s what it is. To make our solvent a little tastier, we’ll dissolve in some delicious sugar. The sugar is the solute. Just to keep track, we now have water (solvent) that we’ve dissolved sugar (solute) in, to make sugar water (our solution).
Now that we have our solution of sugar water, we’ll grab a beaker shaped in a u-shape. Right in the middle of the tube, imagine a bit of Gore-tex that cuts the U in half. Gore-tex is our “semipermeable membrane.” Gore-tex is a thin plastic, dotted with a billion tiny little holes that allow water vapor to pass through, but liquid to stay out.
In one arm of the U-tube, we pour our sugar water mixture. On another we pour our plain old water. That’s when the magic of osmosis begins. The level of liquid in the sugar water arm will slowly rise, as the solvent (water) moves through the Gore-tex, to make both sides of the arm more equal in a sugar-to-water ratio.
But why does that happen? What is the force that drives the molecules through the membrane? This is a misleading question, because there is no real “force” in the physical sense other than the thermal energies all molecules possess. Osmosis is a consequence of simple statistics: the randomly directed motions of a collection of molecules will cause more to leave a region of higher concentration than return to it. Simply put, the molecules wants to find equilibrium and because the one side of the arm is crowded with sugar, it has less water molecules than the other side. So water molecules from the other side decide to move on over to make the concentration of water on each side more equal.

Diffusion and Osmotic Flow
Suppose you drop a lump of sugar into a cup of tea, without stirring. Initially there will be a very high concentration of dissolved sugar at the bottom of the cup, and a very low concentration near the top. Since the molecules are in random motion, there will be more sugar molecules moving from the high concentration region to the low concentration region than in the opposite direction. The motion of a substance from a region of high concentration to one of low concentration is known as diffusion. Diffusion is a consequence of a concentration gradient (which is a measure of the difference in escaping tendency of the substance in different regions of the solution).
You must clearly understand that there is really no special force on the individual molecules; diffusion is purely a consequence of statistics.
Osmotic flow is simply diffusion of a solvent through a membrane impermeable to solute molecules
In the absence of the semipermeable membrane, diffusion would continue until the concentrations of all substances are uniform throughout the liquid phase.
Osmotic Equilibrium and Osmotic Pressure
One way to stop osmosis is to raise the hydrostatic pressure on the solution side of the membrane. This pressure squeezes the solvent molecules closer together, raising their escaping tendency from the phase. If we apply enough pressure (or let

the pressure build up by osmotic flow of liquid into an enclosed region), the escaping tendency of solvent molecules from the solution will eventually rise to that of the molecules in the pure solvent, and osmotic flow will cease. The pressure required to achieve osmotic equilibrium is known as the osmotic pressure.
Note that the osmotic pressure is the pressure required to stop osmosis, not to sustain it.
It is common usage to say that a solution “has” an osmotic pressure of “x atmospheres”. It is important to understand that this means nothing more than that a pressure of this value must be applied to the solution in order to prevent flow of pure solvent into this solution through a semipermeable membrane separating the two liquids.
Reverse osmosis
What is Reverse Osmosis?
So we learned that in osmosis, a lower-concentrate solution will filter its solvent to the higher concentrate solution. In reverse osmosis, we are (literally) just reversing the process, by making our solvent filter out of our high concentrate into the lower concentrate solution. So instead of creating a more equal balance of solvent and solute in both solutions, it is separating out solute from solvent.
But as we’ve explored, that isn’t something that solutions really want to do. How do we make reverse osmosis occur?
By applying a hydrostatic pressure greater than the osmotic equilibrium to the high-solute side of an osmotic cell will force water to flow back into the fresh-water side.
In reverse osmosis, we’d have ourselves a saltwater solution on one side of a tank and pure water on the other side, separated by a semi-permeable membrane. We would apply pressure to the saltwater side of the tank–enough to counteract the natural osmotic pressure from the pure water side, and then to push the saltwater through the membrane. (For saltwater this takes about 50-60 bars of pressure). But because of the size of the salt molecules, only the smaller water molecules would make it to the other side, thus adding fresh water to the water side, and leaving the salt on the other.
Where is Reverse Osmosis Used?
The most common uses of reverse osmosis is desalination of water. That includes large plants (there are over 100 countries using desalination) or smaller operations like the one on this site.

Pre-treatment commonly employs activated-carbon filtration to remove chlorine (which damages RO membranes by creating pinhole leaks) if this is present in the feed water and sediment filters to remove organics larger than 5 microns.
Bacteria, pathogens and cysts are unable to pass through semipermeable membranes.
Reverse osmosis is also one of the few ways that we can take certain minerals or chemicals out of a water supply. Some water sources have extremely high levels of natural fluoridation, which can lead to enamel fluorosis (mottled teeth), or the much more severe skeletal fluorosis (an actual bending of a person’s bones or skeletal frame). Reverse osmosis can filter out fluoride, or other impurities, on a large scale in a way that a charcoal based filter (like the one most commonly found in homes) can’t.
It’s also used for recycling purposes; the chemicals used to treat metals for recycling create harmful wastewater, and reverse osmosis can pull clean water out for better chemical disposal.
But even more fun than recycling? Wastewater reverse-osmosis treatments, wherein wastewater goes through the process to create something drinkable. They’ve nicknamed it “toilet to tap” for a reason, and although it might give you pause, it’s a promising ways for developing nations to produce drinkable water.
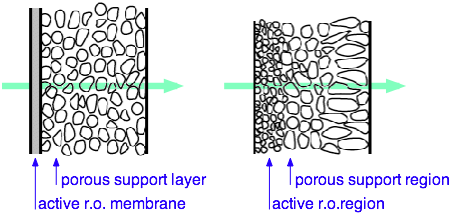
Reverse Osmosis Membranes
The efficiency and cost or RO is critically dependent on the properties of the semipermeable membrane. Membranes intended for fresh water are different from membranes intended for brackish or salt water.

Salt Water vs Brackish Water vs Fresh Water in Reverse Osmosis
As concentration of solute particles increases, the concentration of water molecules per unit volume of solution decreases and vice versa. As solute concentration increases, the osmotic pressure will increase also.
Seawater is very high in total dissolved solids (TDS) approximately 36,000ppm (36,000mg per litre.) Where as the water from the Gasgoyne River near Carnarvon (when we were last there) tested at 1,298ppm (1,298mg per litre).
As you can see the concentration of seawater is much higher than the water from the Gascoyne River and therefore the osmotic pressure is also a lot higher. So the hydrostatic pressure required to rise above the osmotic equilibrium is also much higher.
What this means in a reverse osmosis system, such as the H2O on the Go system, is that the pressure on the water going into the pressure vessel, will need to be adjusted according to the concentration of the solute (feed water).
To rise above the osmotic equilibrium of seawater a pressure of between 50 to 60 bars is required, we recommend using 55 bar (800psi) and you use set this using your needle valve and pressure gauge.
Brackish water is less concentrated than seawater so has a higher concentration of water molecules therefore requires less hydrostatic pressure to rise above the osmotic equilibrium, as all brackish water has a different TDS level the pressure required will vary, it may be as low as say 150 psi or as high as 450 psi.
Water from the Gasgoyne River with a such a low TDS classed as fresh water so has a much higher level of water molecules compared to salt and brackish water. Therefore it requires very little hydrostatic pressure to rise above the osmotic equilibrium.
As you can see, the hydrostatic pressure required will vary depending on the feed water concentration.
So how will you know what pressure is required?
Easy, you just look at your flow meter. Our one membrane system makes approximately 60 litres an hour regardless of the feedwater concentration and our 2 membrane system make approximately 100 litres per hour, again regardless of feedwater concentration.
So you begin operation, as usual, with the needle valve fully open and you gradually increase the pressure until you reach the correct litres per minute for your system. Once this volume has been reached, the system is applying the correct hydrostatic pressure for the concentration of the feedwater you are using.
Simple really!
References
Kershner, Kate. “How Reverse Osmosis Works” 08 May 2008. HowStuffWorks.com. <http://science.howstuffworks.com/reverse-osmosis.htm> 03 February 2015.
This content is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. Permissions beyond the scope of this license may be available at webmaster@H2OontheGo.com.


